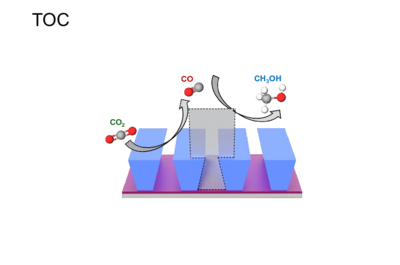
Spatially Patterned Architectures to Modulate CO2RR Cascade Catalysis Kinetics.
Artificial cascades for the electrochemical CO2 reduction reaction (CO2RR) to form value-added products are a developing technology, in which mass transport, reaction rates, local microenvironment and concentrations are all crucial parameters. These properties are highly dependent on the spatial arrangement of active site immobilized onto the electrode surface. However, the design principles for building efficient and selective artificial cascades remain an open question. Reaction-diffusion modeling has emerged as a powerful tool to assess the impact of a particular reaction parameter on a multi-step cascade reaction. Finite element analysis allows us to address this problem by constructing a complete Multiphysics computational model to investigate the geometrical architectures that give rise to more efficient CO2RR cascade systems. A 2D model with periodic boundary conditions was constructed to represent a cross-section of geometric trenches made in a planar cathode. CO2RR to CO (Cat A) was modeled at the bottom of trenches, while the CO reduction reaction (CORR) to MeOH (Cat B) was modeled on the sides and top of trenches. The parasitic hydrogen evolution reaction (HER) was modeled at all cathodic surfaces. The tertiary current distribution (tcd) interface was used to model the electrode and electrolyte domains. Cathodic reactions were modeled with Butler-Volmer kinetics. Bicarbonate equilibrium reactions were considered in the aqueous electrolyte domain. By performing a series of parametric sweeps on the dimensions of the 3D architecture, we were able to screen many trench geometries and identify which structures offered the best cascade selectivity and current densities. In summary, 3D architectures show a dramatically improved performance over planar architectures, which was an expected result due to the improved mass transport of intermediate species to CORR in confined geometries. The use of larger trench depths improved outlfux of MeOH until a depth of ~100um; beyond this point, the increased local pH within the trench shifts the electrolyte equilibrium reactions towards the formation of bicarbonate and depletes the available CO2. At this favorable depth, changing the angle of the trench walls further changes the pH gradient in the local microenvironment. Interestingly, despite similar total current densities for Cat B, a confined trench configuration significantly suppressed the HER compared to an open configuration. Our model attempt to find a balance between mass transport limitations, spatial patterned architectures, side reactions and reactants feedstock.