A COMSOL Multiphysics® Software Interface with GEMS3K for Modeling Reactive Transport (Geo)Chemical Processes
This paper presents a generic interface for reactive transport process modeling that was developed between the COMSOL Multiphysics® software Java API (transport model) and GEMS3K (reaction model). While the transport of different species, kinetics of dissolution/precipitation, system potentials, fluid and gas flow, etc. can be modeled in a complex chemical system with COMSOL® software, the reaction results can be updated using the open source C++ (geo)chemical thermodynamic modeling software, GEMS3K at each step of the analysis. GEMS3K is designed based on Gibbs Energy Minimization (GEM) method for a chemical system with advanced thermodynamic features including multi-phase and multi-component computation capacities. A Java Native Interface approach (JNI) was used to transfer GEMS3K C++ methods to Java and connect to the COMSOL® Java API. An Operator Splitting technique was used to manage two software during the analysis. The developed interface can be used for a wide range of multiphysics (geo)chemical problems with available GEMS-format thermodynamic databases (e.g. CEMDATA for cement). The current version of the interface is one dimensional but extensions to three dimensional studies are straight forward.
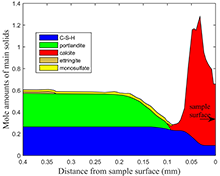
The developed interface was used to model the cement degradation due to carbon dioxide exposure in extreme down-hole pressure and temperature conditions. According to the experimental studies, cubic class H cement samples were wet cured for 28 days in Mt. Simon brine and then exposed to carbon dioxide in different scenarios of temperature and pressure. The degradation of the cement samples due to acidic brine medium from dissolved carbon dioxide were modeled using the developed interface. As an example, the results of the models are shown as solid phase diagrams at selected times for high temperatures (T=85 C) in Figs. 1 and 2 at initial exposure (age of 28 days) and 42 days after exposure (age of 70 days). While the small amounts of dissolved carbon in Mt. Simon brine caused the formation of a calcite layer in the sample surfaces (Fig. 1), the 42 day exposure to carbon dioxide caused the complete dissolution of calcium hydroxide in the hydrated cement and formation of high amounts of calcite layer very close to sample surface. The longer exposures result in the movement of this calcite layer more into the sample; therefore, lead to a high porosity medium on the sample surface with amorphous silica left.
下载
- jafari_presentation.pdf - 1.3MB
- jafari_abstract.pdf - 0.47MB



