A Multifaceted Model Exploring the Role of Mucus and Shear Stress in Intestine
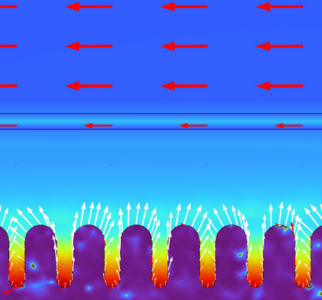
The intestine, a muscular, tubular organ within the digestive system is responsible for the final stages of food processing. The cyclic material transport generates a shear stress on the intestinal lining known as epithelium. Recently, the application of shear stress on epithelial cells has been shown to affect cellular processes such as proliferation, differentiation, and mucus secretion. Despite the extensive research on biological regulation and functional pathways, and biomechanical analyses identifying mechanical events, the mechanical role of mucus and the effect of shear stress in these processes are not fully understood. Our approach is based on a three-phase field and a fluid-solid interaction method to represent the basic biomechanical microenvironment of the intestine. We used experimental data on colonic pressure, mucosal and fecal rheology, and crypt characteristics to predict the shear distribution in the epithelium. Our model provides important insights into the time-space mosaic of mechanical forces, as well as how mucus thickness affects the spatial shear stress distribution. Our results suggest that increasing thickness results in a lower shear stress delivery. In all cases we analysed (100-800 µm) shear stress was significantly lower compared to the total absence of mucus. At the crypt base, we did not see a significant difference among different mucus thicknesses. However, on cell migration route and crypt top yielded significant differences. We observed a similar trend at the crypt top and migration route, albeit at the crypt top the variation was more drastic among mucus groups. Our model allows studying the effect of various properties such as viscosity, density, and geometry to investigate possible mechanistic aspects of various intestinal processes in homeostasis and disease. To validate these findings, we developed a microfluidic device with intestinal cells that could allow generating shear stress and mapping the cellular forces. Using our on-chip model we aim to explore the interplay among shear stress, crypt dynamics, and mucus.
下载
- erbay_paper_comsol_florence_2024.pdf - 0.48MB
- erbay_8501_poster.pdf - 2.44MB
- 4_ibrahim_erbay.pdf - 3MB
