A Wall-Cooled Fixed-Bed Reactor Model for Gas-Phase Fischer-Tropsch Synthesis
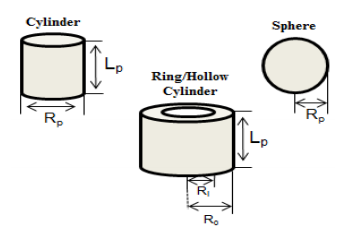
In the early 1920’s, Gas-To-Liquids (GTL) and Coal-To-Liquids (CTL) technologies were developed to account for the depleting crude oil resources [1]. During this period, Franz Fischer and Hans Tropsch developed a process to convert synthesis gas (syn gas), derived from coal gasification, to a wide range of high value-added products. This process later came to be known as Fischer-Tropsch (F-T) synthesis [2]. F-T synthesis was an experimental success but its economic viability became a topic of concern as refining of crude oil was a more developed and an economically attractive option. The syn gas used in F-T synthesis can be derived from various feed stocks such as coal, natural gas, biomass, and waste. This indirect liquefaction process, also termed as feed-to-liquids, is often referred to as XTLs (Where X: C=coal, G=natural gas, B=biomass, W=waste) [3]. Multi-Tubular Fixed Bed Reactors (MTFBR) and Slurry Bubble Colum Reactors (SBCR) are widely employed for FTS. An MTFBR is similar to a shell and tube heat exchanger with a catalytic reaction taking place on the tube-side and a typical MTFBR contains about 10 to 50,000 tubes. A coolant, such as water or a molten salt, flows on the shell-side to control the catalyst bed temperature within proper design limits. To model such a system, detailed knowledge about shell-side fluid-solid interactions coupled with tube-side fluid-solid transport-kinetic interactions is required. The primary objective of this study is two-fold: (1) Analyze the performance of different catalyst particle shapes (sphere, cylinder & ring) on a catalyst scale; (2) Use the catalyst pellet results and analyze the catalyst performance on a reactor scale. The micro kinetic olefin readsorption model proposed by Wang et al. (2003) for a Fe-based catalyst was used as the basis for development of the particle-scale transport-kinetics model. The formation of linear olefins and paraffins up to carbon number of 20 are described by a reaction network that consists of 43 species that participate in 40 reactions, which includes the water-gas shift reaction. The phase behavior of the FT reaction products are described using the Soave-Redlich Kwong equation of state. The coupled system of nonlinear ODE’s are solved using COMSOL Multiphysics® software. A 1-D axial dispersion model with finite heat and mass inter-particle film resistances was used to describe species transport in the gas bulk. The Ergun equation was used to compute the gas-phase pressure drop. To obtain the observed rate on the particle-scale, the overall effectiveness factor was calculated from the integral rate at each grid point on the boundary that was coupled to the reactor geometry using extrusion coupling. The 2-D particle domain was meshed using triangular elements and the coupled boundaries were meshed with equal number of elements. The concentration profiles of the species in a typical Fe-based spherical catalyst pellet along the dimensionless reactor bed length and the concentration profiles of key components in the fixed-bed under typical process conditions are shown in Figure-1.
下载
- nanduri_presentation.pdf - 1.89MB
- nanduri_poster.pdf - 1.26MB
- nanduri_paper.pdf - 0.52MB
- nanduri_abstract.pdf - 0.11MB
