Analysis of PhotoThermal Ablation
Cancer cells have an increased sensitivity to heat compared to healthy cells; at 42°C cancer cells are destroyed while normal cells can survive up to 47°C. Thus hyperthermia-based approaches that target specific spatial locations and simultaneously provide controlled thermal exposure represent an important opportunity for future treatment protocols. Current limitations in available hyperthermia induction methods and tumor accessibility have prevented the widespread implementation of this therapy. However, laser induced hyperthermia, termed PhotoThermal Ablation (PTA), allows tumors sensitized by the presence of gold nanoparticles to be selectively exposed to radiation in the infrared regime. This nanoparticle assisted PTA has shown considerable promise in laboratory controlled, small animal studies, but needs to be further refined for use in human or veterinary clinical practice.
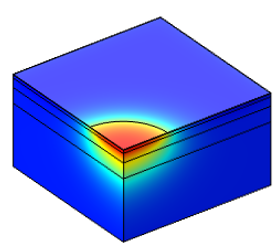
In PTA therapy, interaction of the laser light with the gold nanoparticles produces local heating that causes irreversible damage to the targeted tissue. The PTA thermal profile is directly related to the irradiance distribution of the incident radiation. To provide a better understanding of PTA, a predictive physics-based computational model of light diffusion in tissue has been developed. The time dependent diffusion of light is solved in COMSOL Multiphysics® simulation software using the equation for radiative transfer implemented as a General Form PDE. Results of the implementation have been verified by comparison with predictions of an analytical solution for an infinite homogeneous medium. The model has been extended to predict light diffusion in a layered tissue structure consisting of Epidermis, Dermis, Sub-cutaneous fat and Muscle. Transient temperature distributions through the layered tissue structure are predicted from the balance of heat transfer due to light absorption, heat dissipation due to perfusion in the active tissue layers and losses due to convection from the outer surface.
下载
- de_taboada_paper.pdf - 1.19MB
- de_taboada_biosci_presentation.pdf - 0.86MB



