COMSOL Simulation of Transcutaneous O₂ Transport for Accurate Personalized Respiratory Monitoring
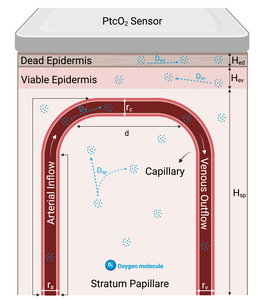
Accurate, real-time measurement of arterial oxygen partial pressure (PaO₂) is essential for managing respiratory diseases and critical care conditions. Traditional arterial blood sampling for PaO₂ is invasive, painful, and provides intermittent snapshots rather than continuous monitoring [1]. Transcutaneous oxygen partial pressure (PtcO₂) monitoring offers a promising, non-invasive alternative currently employed clinically as an indirect measure, typically interpreted within a reference range rather than as an absolute value. However, reliance on PtcO₂ alone has significant limitations, including physiological variability, skin temperature sensitivity, and environmental influences, thereby limiting its standalone clinical utility [2]. Precisely correlating PtcO₂ measurements with PaO₂ is thus critical for enhancing the clinical accuracy and utility of transcutaneous oxygen monitoring devices. This study introduces a detailed computational modeling approach using COMSOL Multiphysics software to simulate oxygen transport from arterial blood through multilayered skin structures. Inspired by Grossmann’s microcirculatory capillary loop model [3], our finite element model (FEM) characterizes oxygen transport through three distinct skin layers: the dead epidermis, viable epidermis, and the vascular-rich stratum papillare. COMSOL’s Laminar Flow module was utilized to simulate blood flow within capillaries, while the Transport of Diluted Species module addressed oxygen diffusion through tissue layers, incorporating physiological phenomena such as nonlinear oxygen-hemoglobin dissociation, metabolic oxygen consumption, and temperature-dependent variations. Simulations systematically examined physiological responses under conditions of blood flow occlusion and oxygen inflow variations, reflecting clinical scenarios like hypotension and hypoxia. Results demonstrated how simulated variations in PtcO₂ levels can be effectively correlated with PaO₂ changes, enhancing the clinical utility of PtcO₂ as a reliable parameter for respiratory monitoring. Furthermore, simulations incorporating personalized variations—such as changes in skin thickness and oxygen diffusion coefficients—demonstrated significant sensitivity to these parameters, highlighting the model’s capacity for individualized respiratory monitoring. Our computational approach validates the effectiveness of COMSOL Multiphysics in accurately modeling the complex dynamics of transcutaneous oxygen measurement. By establishing robust correlations between simulated PtcO₂ and clinically observed PaO₂, the model supports enhanced accuracy and clinical relevance of wearable monitoring devices. Future integration of additional personalized physiological parameters—like metabolic rates, skin permeability, hydration, and localized temperature conditions—promises significant advancements in personalized respiratory monitoring, ultimately improving patient outcomes in clinical and home-care settings.