Fluid Dynamics & Electrochemical Kinetics in Annular Reactors: A Study for Resource Recovery
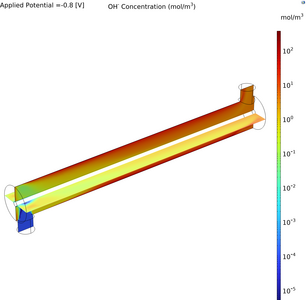
Sustainable wastewater management has stimulated the exploration of innovative technologies, such as electrochemical nutrient recovery, which promises efficient recovery and reuse of vital nutrients, reducing environmental impacts and resource wastage. This study presents the modeling of an electrochemical annular plug flow reactor using magnesium (Mg) as a sacrificial anode, aimed at enhancing the understanding of fluid dynamics, mass transfer and electrochemical kinetics within the system. A continuous reactor ensures steady, controlled operations and enhanced reaction kinetics, which are critical for scalable applications. In addition, leveraging an annular space provides a high surface area to volume ratio for electrochemical reactions, improving efficiency and throughput. Finally, the Mg sacrificial anode facilitates the reactions that recover nutrients (phosphorus and nitrogen) by promoting the formation of solid products that can be easily separated from the liquid. Anode reaction: Mg → Mg^(2+)+2e^(-) Bulk reaction: Mg^(2+) + NH4^(+) + PO4^(3-) + 6H2O →MgNH4 PO4.6H2O(s) Employing COMSOL Multiphysics, this research study conducts detailed computational fluid dynamics (CFD) simulations and electrochemical modeling to analyze fluid transport and tertiary current distributions within the reactor. The configuration of the annular reactor is designed with the inner solid cylinder serving as the Mg anode and the outer hollow cylinder as a stainless-steel cathode. Laminar flow conditions are assumed, reflecting the controlled fluid dynamics within the annular space. The electrode kinetics are described using the Butler-Volmer equation, providing a detailed simulation of the electrochemical processes under various operating conditions. Employing steady-state analysis, the study aims to conduct parametric studies to examine how various operational parameters such as inlet velocity and applied cell voltage affect Mg, hydroxide and current distributions.