Investigation of Performance of SOFC in Hydrocarbon Fuel
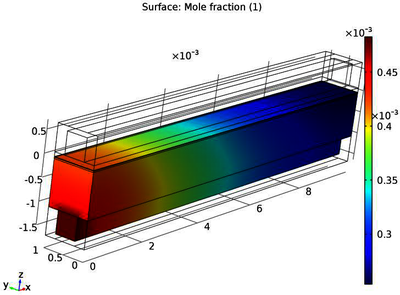
SOFC is a high temperature electrochemical device known for its fuel flexibility. Apart from using pure hydrogen, it can utilize CO (carbon monoxide), CH4 (methane) or any other higher hydrocarbon. Since methane is highly researched hydrocarbon fuel, it was chosen to start with. The most prominent problem faced while using hydrocarbon fuel in SOFC is the formation and deposition of carbon on the nickel catalyst, which hampers the efficiency of the cell by occupying the much needed active sites. Further, the carburization of anode leads to disintegration of anode by metal dusting and is of utmost importance to avoid it. Steam reforming is known to produce high concentrations of hydrogen in the product. It was found that with increasing reforming ratio, carbon formation decreases. The objective of the present study is to simulate the performance of SOFC with the reformed feed (CH4+Steam) using COMSOL Multiphysics® software. In the present work, equilibrium composition of a hydrocarbon fuel was derived using a free source CEA software. Having the right composition, a model was developed that incorporated hydrocarbon fuel. COMSOL® software has an in-built material model of an SOFC that by default uses pure hydrogen as fuel and air on the cathode. After understanding the basic working and analysis, hydrocarbon fuels were incorporated. A lot of parameters had to be taken care of because instead of pure hydrogen, a reformed product enters the fuel chamber (mixture of CH4, CO, CO2, H2, H2O) at a fixed temperature of 1073K. Thus, SOFC performance analysis was carried out with reformed fuel. Also, the reactant concentration and carbon activity profiles were generated across the fuel channel. Similar procedure can be expanded to heavier hydrocarbons such as dodecane if the necessary data is available.
下载
- kumar_presentation.pdf - 1.46MB
- kumar_abstract.pdf - 0.15MB
