Modeling Transient Adsorption/Desorption Behavior in a Gas Phase Photocatalytic Fiber Reactor
Integration or retrofitting of photocatalytic air purifying units into HVAC (Heating, Ventilation and Air Conditioning) equipment is an interesting approach for abating indoor air pollution and removal of volatile organic compounds. An attractive possibility is the use of glass fiber filter mats, coated with a photocatalyst. The thin, long fibers not only offer the advantage of exposing a large catalyst surface area, but the open structure also enables sufficient light penetration, limited pressure drop, and silent operation conditions while still exerting sufficient filtering capacity.
Determining the kinetic parameters of photocatalytic systems is an important step in the design and development of efficient air purification units for integration in HVAC systems. Determination of photocatalytic kinetic parameters is relatively straightforward for batch processes, but is somewhat more difficult for continuous flow systems, unless steady state conditions are attained. During operation, mostly transient behavior is observed until steady state under a given set of conditions is reached. In this work we used COMSOL Multiphysics for accurate modelling of the pollutant concentrations in these transient zones in the case of acetaldehyde adsorption on TiO2 coated glass fiber filters in dark conditions. This results in useful parameters such as the adsorption/desorption rate constants and the maximum adsorptive capacity of the filter. It thus provides vital information for the design and development of photocatalytic air purification units, since adsorption of pollutants is an essential precursory step in photocatalysis.
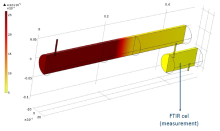
Adsorption experiments were performed in a borosilicate glass tube reactor (diameter of 5 cm and length of 44 cm) (Figure 1). Acetaldehyde was used as model compound for indoor air contamination. It was mixed with clean air using mass flow controllers and dosed to the reactor set-up at inlet concentrations that were varied between 20 and 220 ppmv at a fixed effective total gas flow rate of 400 cm3 min-1. The concentration of acetaldehyde was monitored on-line using FTIR spectroscopy. The Langmuir adsorption/desorption kinetic parameters were derived from analysis of a series of adsorption experiments, and from a COMSOL Multiphysics model. A transient model was used and adsorption and desorption reactions were included as a volumetric reaction. For determining the kinetic parameters (adsorption and desorption rate constants and the maximum adsorptive capacity of the filter) from the Comsol model, the time-dependent optimization module was used in conjunction with the calculations.
Despite the differences between both approaches, similar adsorption/desorption kinetic parameters were found. The advantage of the modelling/optimization approach is that the latter could yield the parameters simultaneously from a limited number of experiments. The obtained parameters can be used to model the spatial variation of the adsorption/desorption reaction rates and concentrations throughout the reactor (Figure 2), even when time varying boundary concentrations were assumed.
下载
- denys_presentation.pdf - 1.66MB
- denys_poster.pdf - 0.41MB
- denys_abstract.pdf - 0.12MB



