Simulation-Based Sensitivity Analysis of the Direct Ammonia Fuel Cell Operating Conditions
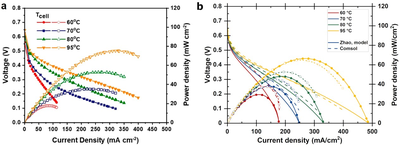
Ammonia is studied both as a hydrogen carrier and as a fuel for renewable energy infrastructure. An important advantage of ammonia compared to carbon-based fuels is that it can be oxidized without greenhouse gas emissions, producing only N2 and water. Direct ammonia fuel cells (DAFCs) use ammonia as a fuel and their theoretical voltage is almost equal to hydrogen fuel cells (HFC; 1.17 V vs 1.23 V at 25 °C and 1 bar). Different from HFCs, most DAFCs in literature use liquid anode feed because highly concentrated aqueous ammonia solutions are commonplace and, so far, have yielded higher power than gaseous anode feed. However, gas-fed DAFCs are also studied and their operation could benefit from simpler balance of plant and operation. The ammonia oxidation reaction (AOR) kinetics are the main voltage loss component of DAFCs, significantly slower than the hydrogen oxidation reaction in HFCs, or even the oxygen reduction reaction (ORR). Therefore, in similar operating conditions, a DAFC generates lower voltage and power than a typical HFC. Research and development of the DAFCs is therefore needed to make it a viable technology, and simulations using established FC physics can be a very useful tool in this process. We studied the operation of DAFCs with COMSOL Multiphysics® and one-dimensional cross-section models using the Fuel Cell & Electrolyzer Module. Gas convection and diffusion inside the porous electrodes were simulated using Darcy’s law and Maxwell-Stefan diffusion in the Transport of Concentrated Species node, respectively. Transport in liquid electrolyte was simulated with the Tertiary Current Distribution interface, and the ionomer and anion exchange membrane (AEM) conductivity and water transport with Secondary Current Distribution and Transport of Diluted Species interfaces, respectively. We used analytical functions from literature to parameterize the AEM and the ionomer properties. The electrochemical reactions, AOR and ORR, were included as reactions in a porous electrode, using thermodynamic data from the NIST-JANAF thermochemical tables for the standard reaction potentials and literature data for the kinetic parameters. As could be expected based on literature, temperature, relative humidity, cell pressure, and the ammonia and hydroxide concentrations of the liquid anode feed were the most important parameters for the DAFC performance. In most cases, the AOR kinetics were affected the most and quite often the anode ionomer losses changed more than the ORR kinetic losses, despite the latter being a larger voltage loss. Improving the reaction kinetics naturally enhances the performance but, in some scenarios, when increasing catalyst loading, increased ionomer transport losses could counteract the reduced kinetic overpotential, leading to only small net improvements. While perhaps not unexpected or surprising, our results help to understand the DAFC operation and the impacts of different operating parameters, and to direct and prioritize research and development to the most important aspects of the DAFCs. This research was done under the TELEGRAM project. This project has received funding from the European Union’s Horizon 2020 Research and Innovation programme under grant agreement No 101006941. The project started on the 1st of November 2020 with a duration of 42 months.
下载
- Kemppainen_4761_presentation.pdf - 9.22MB
