Uniform Reaction Rates and Optimal Porosity Design for Hydrogen Fuel Cells
We develop a porosity-optimization problem to improve the electrochemical reactions taking place in hydrogen fuel cells. We introduce a mathematical model, which involves a system of conservation laws defined in a porous space domain. Our goal is to find the domain's optimal porosity function that can make the oxygen-hydrogen reaction as uniform as possible. The optimal porosity design contributes to solve water and heat accumulation problems in fuel cells.
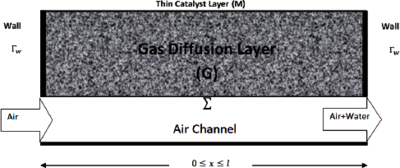
INTRODUCTION: Hydrogen fuel cells are devices that convert the chemical energy of reactants to electricity, through electrochemical reactions between hydrogen and oxygen gas. Figure 1 shows a two dimensional cross-section of the cathode components of a cell: an air channel, gas diffusion layer (G, a porous medium), catalyst layer (M), which is taken as a thin boundary.
The oxygen in air flows from the air channel to domain G, and on M it reacts with hydrogen forming water, heat, and electrons. This electrochemical reaction (1) is not uniform on M, and this causes water and heat accumulation, which reduce the cell's efficiency. Can this reaction be made uniform on M? We show that it is possible by designing domain G with a space-varying porosity.
The gas fluid dynamics in G is governed by the above conservation laws of masses and momentum, modeling the oxygen concentration and the gas mixture pressure and velocity. We show that the porosity of domain G can be redesigned to make a uniform reaction, which build more efficient and longer life fuel cells.
The optimal porosity function that makes the rate of the oxygen-hydrogen reaction (1) as uniform as possible on M is a minimizer of the cost functional E, where the oxygen concentration is obtained from the solution of the above state equations and boundary conditions.
USE OF COMSOL MULTIPHYSICS® SOFTWARE: The above optimization problem is solved using the COMSOL Multiphysics® Software. The differential equations and boundary conditions are written in weak formulation by the user using the GUI. The optimal porosity function is found using the iterative gradient descent method.
RESULTS: The optimal porosity is found through the iterations n=0,1,…,10. In Figure 4, top, for n=0, is taken constant, the corresponding oxygen mass fraction, in Figure 4, bottom, has a large variation relative to that in case n=10. The optimal porosity design is given by , which increases from 0.42 to 0.65. With this porosity design, the corresponding oxygen mass fraction (proportional to the reaction rate) is almost constant over boundary M, as shown in Figure 4, bottom.
CONCLUSION: We solved a porosity-design problem for hydrogen fuel cells to optimize the cathode electrochemical reaction, that makes the cell more efficient.
下载
- alsmail_presentation.pdf - 0.99MB
- alsmail_abstract.pdf - 0.34MB



